Students Break Down Atomic Structure in Science
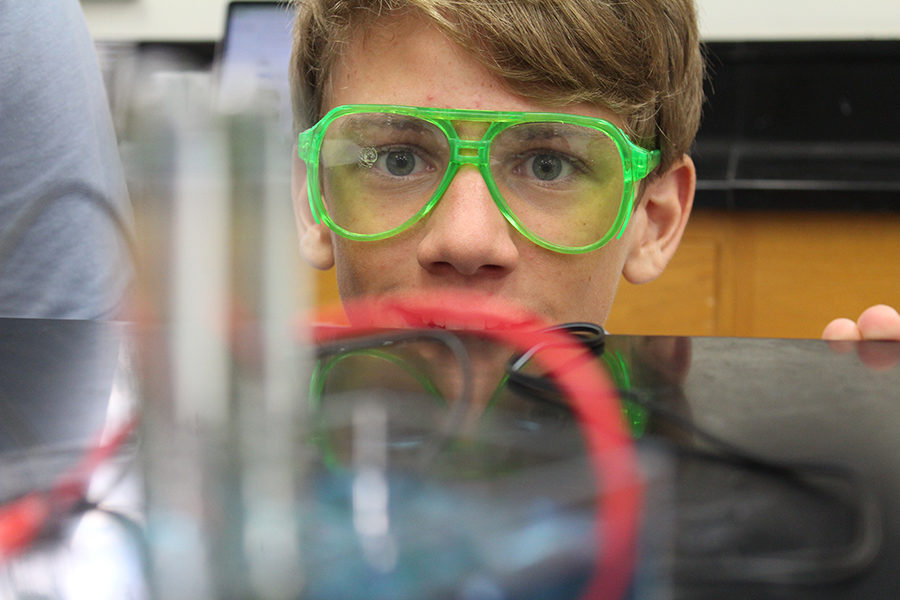
Eighth grader Cole McIlvaine watches as the water is turned into separate gases within his group’s test tubes during his SEAT class.
With hair up and goggles on, eighth-grade students recently had a hands-on experience doing something one can’t do with one’s hands: separating out the hydrogen and oxygen atoms of water. The electrolysis of water lab was performed in eighth-grade science block classes and allowed students to separate the hydrogen and oxygen atoms with the help of electricity. Students then got to play with fire at the end of the lab. Pretty cool, huh?

THe hydrogen gas (on the right) fills the test tube more quickly as opposed to the oxygen because there are twice as many hydrogen atoms in water.
“[The students have] been studying molecules and the chemical makeup of molecules, and with a water molecule – it’s two parts hydrogen to one part oxygen. So it’s nice for students to be able to see that happen in front of them,” said Science Department Chair Ms. Gabriele St. Martin who, along with Ms. Sara Featherston, led the experiment in their science classes “They take water, which is a liquid, and from that they form two gases in a two to one ratio,” St. Martin said.
In this lab, students filled up two test tubes with water and flipped them upside down. A small pole with an electrical current was placed in the mouth of each of the test tubes. Slowly, when the electrical charge was turned on, the hydrogen separated from the water in one tube and the oxygen separated from the water in the other. The electrical current inside the test tubes broke apart the covalent bonds of the hydrogen and the oxygen. In terms of chemistry, a bond is simply an electrical force linking atoms. One wire was a cathode and the other was an anode. The cathode was a positively charged wire and the anode was negatively changed. The hydrogen separated into the cathode since it is a positively charged ion, and the oxygen filled the anode tube because it is a negatively charged ion. Students observed that the hydrogen separated twice as quickly as the oxygen because there is twice as much hydrogen than oxygen in water (H2O).
This lab was not only a fun experience, but provided students a visual regarding how molecules form and how they separate. “The lab helped me get a deeper understanding of the separating of atoms and seeing it helped me understand more about the process of covalent bonding,” said eighth grader Zach German. “It was really helpful for me to do this lab before the test so I could have a visual about how bonds really work.”
“In science, we have been learning about chemistry and how atoms bond in different ways,” added eighth grader Lauren Straub. “We’ve learned about covalent and ionic bonds, and a lot about the periodic table.” Although students have been learning about the basics of chemistry for much of the first semester, via presentations and illustrations, having a physical representation of it really helped students comprehend bonding.
Along with helping students understand the separation and bonding of atoms, the experiment also helped students understand chemical formulas. “The chemical formula for water is H2O, so

Eighth grader Jane Boyland attempts to make a mini explosion by placing a lit match beneath her test tube full of hydrogen.
there are two hydrogen molecules for every oxygen molecule,” said eighth grader Ryan Casey, “Doing the [electrolysis of water] lab helped show that there really is twice as much hydrogen as oxygen in water.” Labs give students a visual representation of what they are learning, and this lab especially showed students something that usually cannot be seen with the naked eye. For some students, though, the exciting part was, of course, playing with fire.
“My favorite part about the lab was the explosion,” said eighth grader Jakob Kroll. “At the end of the lab, we took the test tube of hydrogen and put a lit match [underneath] it and it exploded.” The “explosion” Kroll referred to was more of an audible “pop,” but The Neersyde won’t rain on his pyrotechnic parade. “Waiting for the hydrogen to form [in the test tube] was worth it because of the explosion,” he said.
Along with causing a chemical reaction with the hydrogen and fire, there was a reaction with the oxygen and fire as well. “At the end of the lab, we got the oxygen and put a match under it after it almost went out, and [the oxygen] lit the match back on fire,” said eighth grader Jenny Maciejko.“That part was pretty cool, just like the hydrogen and when we lit that on fire,” she said.
One lab, two elements, two gases, and one “explosion.” What more could any student want?





Jakob Kroll • Dec 6, 2017 at 1:43 pm
Aye they got me up in this article! Also this is a good article.